SAGICO TO RELEASE CLINICAL OUTCOMES DEMONSTRATING SAFETY AND EFFICACY OF A 5° EXPANDABLE INTERBODY ACDF DEVICE
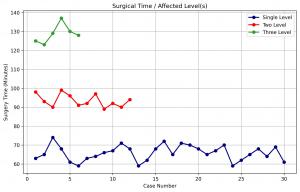
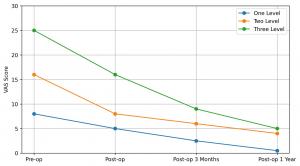
Key conclusions to the literature include zero reoperations, no noted implant failures, and no device-related complications were observed in the findings.
This implant was designed to provide stand-alone stability, eliminating the need for anterior plating, while maintaining strong clinical and radiographic results”
TAMPA, FL, UNITED STATES, February 11, 2026 /EINPresswire.com/ -- SAGICO TO RELEASE ONE-YEAR CLINICAL OUTCOMES DEMONSTRATING SAFETY AND EFFICACY OF A 5° EXPANDABLE INTERBODY CERVICAL DEVICE — James Gibson, President
A newly completed clinical evaluation by SAGICO and its global network of companies and partners, is set to be released. The study will aim to demonstrate that SAGICO’s proprietary 5° expandable cervical interbody implant, with bi-directional internal fixation, delivers safe, effective, and efficient outcomes in patients undergoing anterior cervical discectomy and fusion (ACDF). The unique design was created as a stand-alone implant for use in cervical interbody fusion. Over the year, advances to the implant and various inserter options have improved, to specifically include additional blade depth deployment into the inferior and superior bone penetration.
The implant, is quickly becoming favored by many physicians as it offers one of the largest platforms for the introduction of bone graft material available in the marketplace. James Gibson, SAGICO’s President said “This implant was designed to provide stand-alone stability, eliminating the need for anterior plating, while maintaining strong clinical and radiographic results.” Future clinical literature to be released by SAGICO, will demonstrate the documented success of fusion utilizing radiographic imaging. SAGICO is registered with ClinicalTrials.gov and is utilizing radiographic imaging to confirm stable implant positioning and restoration of cervical lordosis in a prospective data collection study.
The SAGICO 5° Expandable ACDF Implant, when following the proper surgical technique, does not extend beyond the confines of the anterior and posterior intervertebral disc space. Thereby limiting the risk of damage to adjacent vessels and other soft tissues. The bladed implant has unique internal fixation mechanics, once deployed, does not interfere with adjacent implants in a multi-level surgical case. Additionally, the implant may also be used in adjacent levels to a prior fusion, a cervical plate or other implanted hardware.
A key aspect of the surgical outcomes noted a correlation between a decrease in dedicated operating room usage and the reduction on patient exposure to anesthesia. Multiple peer reviewed clinical references have noted that reducing anesthesia time directly enhances patient safety by lowering risks of postoperative complications, such as nausea, infections, thrombosis, and cognitive dysfunction. Key conclusions to the literature include zero reoperations, no noted implant failures, and no device-related complications were observed in the findings.
ABOUT SAGICO
Spinal Analytics & Geometrical Implant Co., LLC (SAGICO) was founded with a purpose to bring years of experience to the medical device industry and offer spinal solutions. SAGICO continues to advocate for innovative technologies designed to advance spinal care and improve patient outcomes. For more than ten years, SAGICO implants and spinal restoration devices have been used in thousands of procedures across Europe, Asia, and the Middle East. Through an extensive global distribution network spanning nearly 60 countries, SAGICO collaborates with academic institutions and medical facilities worldwide to bring forward-thinking advancements to market. The company works in parallel with surgeons to address intractable pain while delivering meaningful benefits to patients. SAGICO’s leadership team has extensive experience obtaining U.S. FDA clearances and securing international regulatory approvals to support the global import and export of medical devices. The company’s mission remains focused on innovation, responsible product development, and achieving the highest levels of patient and physician satisfaction.
MORE ABOUT SAGICO
At SAGICO, we promote the efficiency required to bridge the gap between cost containment and optimal clinical outcomes. We recognize that patient care should remain the primary focus of clinicians—not procurement complexity. Healthcare supply chains often involve competing priorities among providers, administrators, and procurement teams. SAGICO’s hospital and facility procurement services are designed to align all stakeholders toward shared goals, enabling organizations to reduce redundancies, eliminate waste, and enhance performance in today’s value-based care environment.
By promoting efficiency across the healthcare supply chain, SAGICO helps facilities unlock meaningful cost-reduction opportunities while ensuring all products meet or exceed regulatory requirements.
SAGICO and its principals have achieved global success across more than 60 countries. The company operates through a privately held global network with an active pipeline of innovative products and solutions across U.S., European, Middle Eastern, and Pan-Asian markets.
DISCLOSURE
SAGICO is confident in its products; however, surgeons must always rely on their own clinical judgment when determining the appropriate use of any medical device for a specific patient. All SAGICO products must be used in accordance with the package insert, product labeling, and instructions for use. SAGICO does not diagnose medical conditions or provide medical advice. Surgeons must be appropriately trained prior to using any SAGICO product in surgery. The information presented is intended to highlight the depth and future direction of SAGICO’s product portfolio. Product availability may vary by market due to regulatory requirements and medical practices. Please contact SAGICO for additional information prior to any medical procedures.
LEARN MORE AT: WWW.SAGICO.COM
James Gibson
SAGICO
+1 813-830-3636
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.